Research
Multiscale Mechanochemistry
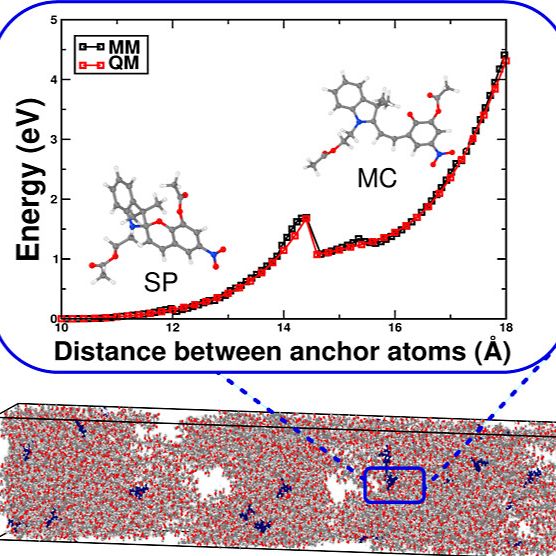
The effect of mechanical force on chemical substances is known as mechanochemistry. Molecules can, for example, be mechanically distorted in an atomic force microscope or an ultrasound bath, thereby changing their spectroscopic properties. This effect is used in mechanochromic materials, which change color when a certain force is exceeded. In our group, we develop multiscale simulation methods for chemical substances under the influence of mechanical force and use these methods to gain a molecular understanding of mechanochemical processes.
Featured Paper:
S. Kumar, B. Demir, A. Dellwisch, L. Colombi Ciacchi, T. Neudecker, “Multiscale Mechanochemical Modeling of Spiropyran-Merocyanine Isomerization in Linear PMMA Polymers”, Macromolecules 2023, 56, 8438-8447.
Molecules Under Pressure
Molecules can be subjected to hydrostatic pressure in a diamond anvil cell. As in the case of mechanical forces, the structural and spectroscopic properties of the molecules change during this process. Changes in the electronic properties of metal complexes, for example, play an important role in the separation, storage and activation of various gases. We develop quantum chemical methods for the calculation of these effects (e.g. HCFF, X-HCFF and GOSTSHYP) and develop materials that perform a desired switching process under pressure. In our review article we present various simulation methods for high-pressure chemistry.
Featured Paper:
R. Weiß, F. Zeller, T. Neudecker, “Calculating High-Pressure Vibrational Frequencies Analytically with the Extended Hydrostatic Compression Force Field Approach”, J. Chem. Phys. 2024, 160, 084101

Emergent Properties
How large does a molecular cluster need to be in order to show the properties of the macroscopic material? This question is surprisingly difficult to answer, as it depends on the chemical system under investigation and the observable to be reproduced. Properties that are encoded at the molecular level but only show up in molecular clusters of sufficient size are called emergent properties. In our group, we investigate emergent properties, e.g., in the field of high-pressure chemistry. A recently published perspective article summarizes different types of emergent properties that are of interest in chemistry.
Featured Paper:
N. Sieroka, T. Lossau, T. Neudecker, "Emergent Properties in Chemistry - Relating Molecular Properties to Bulk Behavior", Chem. Eur. J. 2024, e202303868.

