Magnetic resonance imaging for the study of electroactive biofilms in porous electrodes
The metabolism of electroactive microorganisms enables the use of an external electrode as an electron source or sink. A whole range of possible applications arise from these bioelectrochemical systems, such as the generation of electricity from wastewater or the synthesis of platform chemicals. These and further possible applications are being funded by the German Research Foundation (DFG) as part of the Priority Program SPP 2240 - eBiotech.
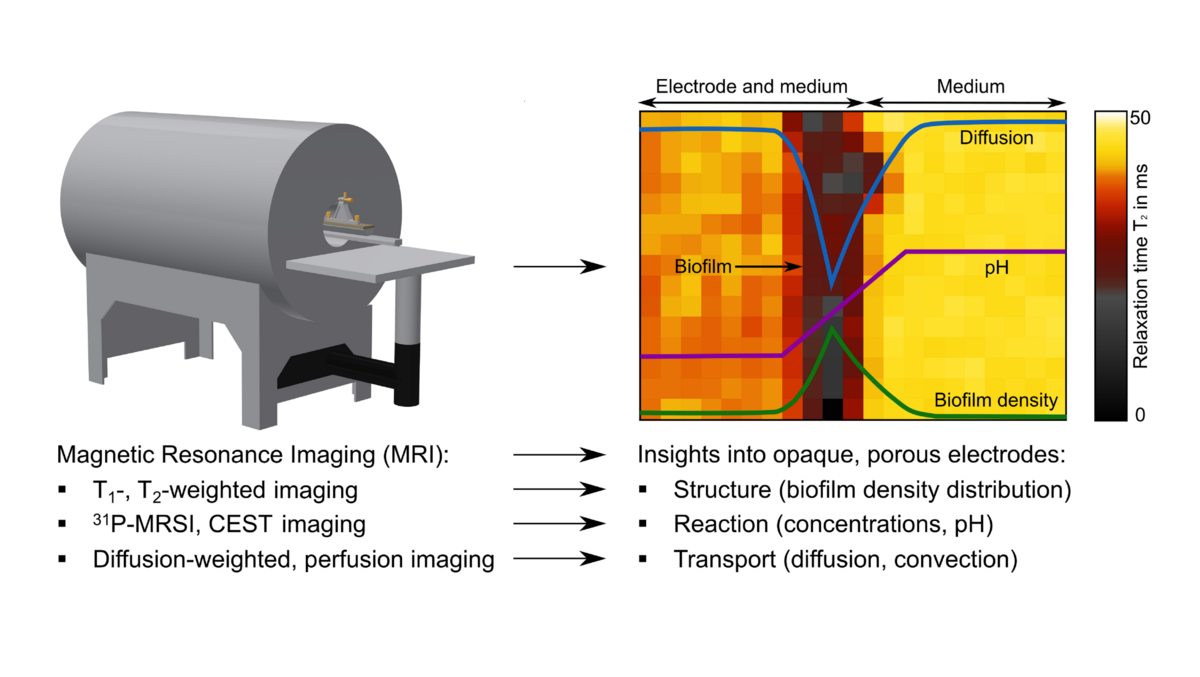
Usually, these electroactive microorganisms form an electroactive biofilm (EAB) on the electrode surface. Porous 3D electrodes provide a large habitat for the EAB and can thus facilitate high metabolic rates and high current densities, which are necessary for the commercialization of bioelectrochemical systems. On the other hand, the biofilm limits the diffusive transport of nutrients and reaction products. In current research, porous electrodes are developed considering the biofilm density, its metabolism and the occurring transport phenomena. In this context models and simulations help to better understand the processes in the electrode-biofilm composite structure. Direct and spatially resolved measurement of parameters such as biofilm density or diffusion coefficients are essential to improve the existing models and evaluate the effectiveness of the porous electrodes. However, the predominant optical methods used at this time fail due to the opacity of the electrodes. Therefore, tools that do not rely on optical transparency are required.
In this project, magnetic resonance imaging (MRI) is applied for the characterization of electroactive biofilms in porous electrodes. Electroactive biofilm structure, diffusive and convective transport, and concentration gradients can be determined non-invasively with an accuracy down to a few micrometers. The in vivo use of MRI to characterize EABs in porous electrodes has not been reported yet. For this purpose, a biofilm reactor compatible to MRI with an internal RF-coil will be designed. The density of different biofilms will be qualitatively determined using T1-, T2-weighted imaging. Diffusion-weighted imaging will provide insight into the coupling of structure and transport. Using qPCR, empirical correlations for quantitative biofilm density as a function of T1-, T2 and the diffusion coefficient as well as its spatial distribution will be derived. Concentration gradients of e.g. nutrients and pH will be determined using a combination of Chemical Exchange Saturation Transfer (CEST) and 31P spectroscopic imaging. The knowledge gained can then be utilized to improve model and to design 3D flow-through electrodes.

![[Translate to English:] Zur Startseite des Fachgebiets Umweltverfahrenstechnik](/fileadmin/user_upload/fachbereiche/fb4/uvt/LOGO/UVT-Logo-Webseite.png)