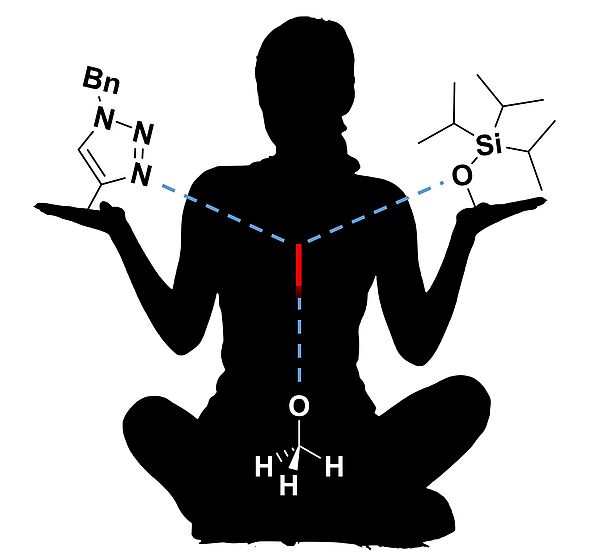
Organische Chemie
Welcome to AG Nachtsheim
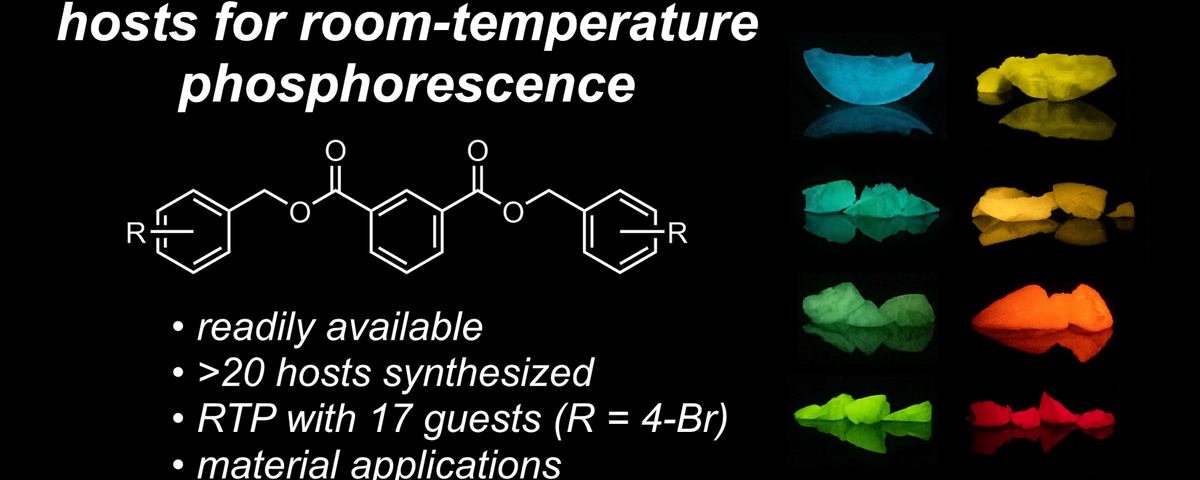
Minimalistic Host-Guest Systems published in Chemical Science
We have discovered that simple organic molecules called dibenzyl isophthalates (DBIs) can act as versatile hosts to create remarkable glow-in-the-dark materials. By incorporating various guest molecules into these DBI hosts, we achieved some of the longest-lasting room temperature phosphorescence reported for metal-free materials - with afterglow effects lasting over 20 seconds. Our DBIs can be easily synthesized and modified to create materials that glow in different colors from blue to red after the lights are turned off. This breakthrough provides a practical and cost-effective alternative to conventional metal-containing phosphors, opening new possibilities for applications in anti-counterfeiting, bio-imaging, and organic electronics. Most excitingly, we found that these materials can even be activated in trace amounts and work effectively when embedded in everyday materials like cotton or dough! Have a look at Chemical Science, 2025, ASAP article https://doi.org/10.1039/D4SC07768G.
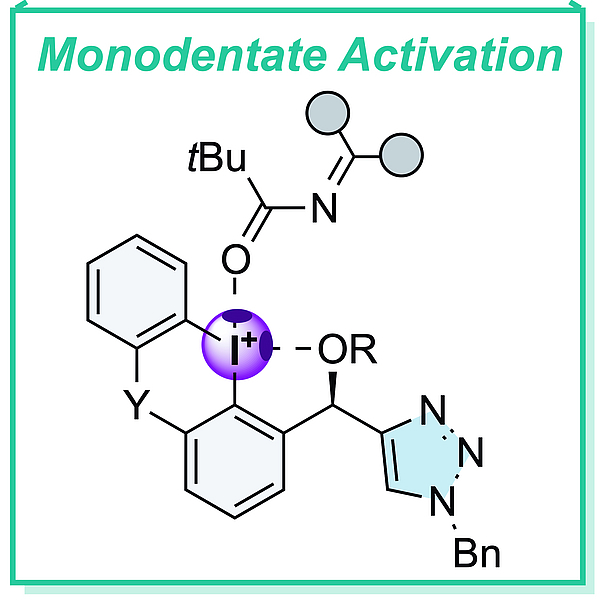
Halchem X, Lodcz 2022
PhD Exam, September 2021
Chemistry - A European Journal
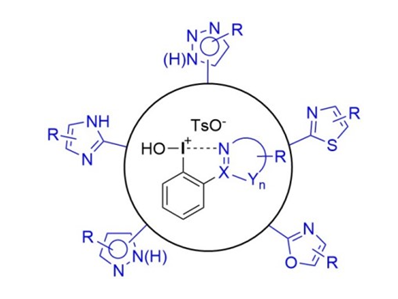
Andreas recent findings about pseudocyclic N-heterocycle stabilized iodanes has just been accepted in Chemistry – A European Journal
https://onlinelibrary.wiley.com/doi/epdf/10.1002/chem.201804957
Congratulation!
Organic & Biomolecular Chemistry
Dominik Göbel M. Sc. received the Thieme Chemistry Poster Prize at the ORCHEM in Berlin (September 2018). Congratulation!
Some of the results are puplished in: http://pubs.rsc.org/en/content/articlelanding/2018/ob/c8ob01072b
Advanced Synthesis & Catalysis
The inside cover picture, provided by Pericàs and Nachtsheim, illustrates the development of novel C1-symmetric iodoarenes as precursors for chiral aryl-λ3-iodanes. The synthesis of these structures is highly modular and allows easy modification on all major substituents, namely the arene, the alkyl ether and the terminal triazole. This is not only the first example for a triazole-based chiral iodoarene but also the so far most efficient C1-symmetric catalyst for the oxidative enantioselective spirocyclization of 1-naphtholcarboxylic acids to spirolactones. C. Hempel, C. Maichle-Moessmer, M. A. Pericàs, B. J. Nachtsheim, Adv. Synth. Catal. 2017, 359, 2931-2941; DOI: 10.1002/adsc.201700246.
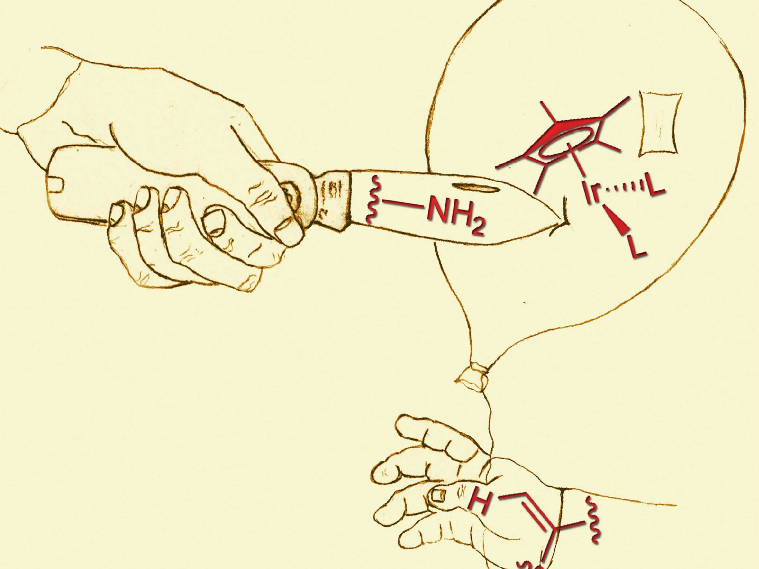
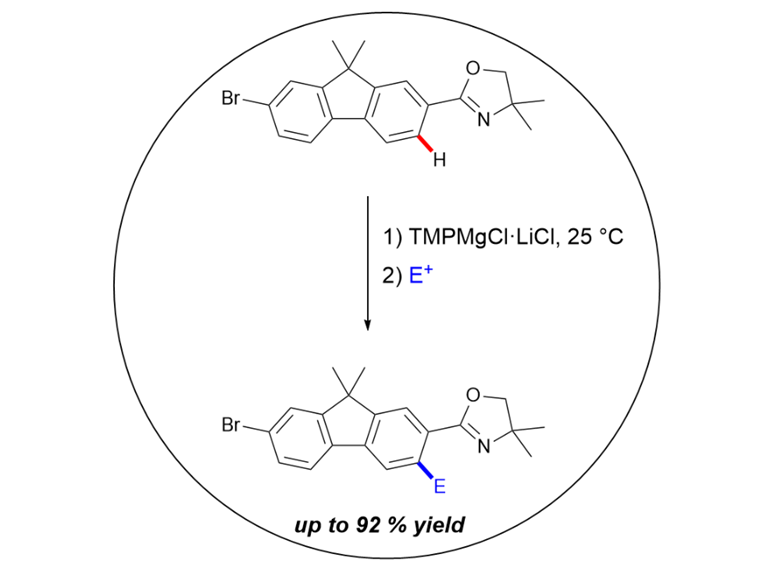
C−H Activation Directing groups are crucial in transition metal-catalyzed C−H activations to control regio-and stereoselectivity. So far, free amines have rarely been used as directing groups since they tend to deactivate the transition metal or undergo undesired side reactions. We demonstrate that free amines in anilines can be used as directing groups in delicate C−H activating alkynylations to give versatile 1,3-enynes in excellent yields and stereoselectivities using highly reactive hypervalent iodine-based alkynylation reagents . L. D. Caspers, P. Finkbeiner, B. J. Nachtsheim, Chem. Eur.J.2017, 23, 2748 –2752; DOI: 10.1002/chem.201781262