Research
Chemistry of Hypervalent Iodine Compounds – From Novel Reagents to Reaction Method Development
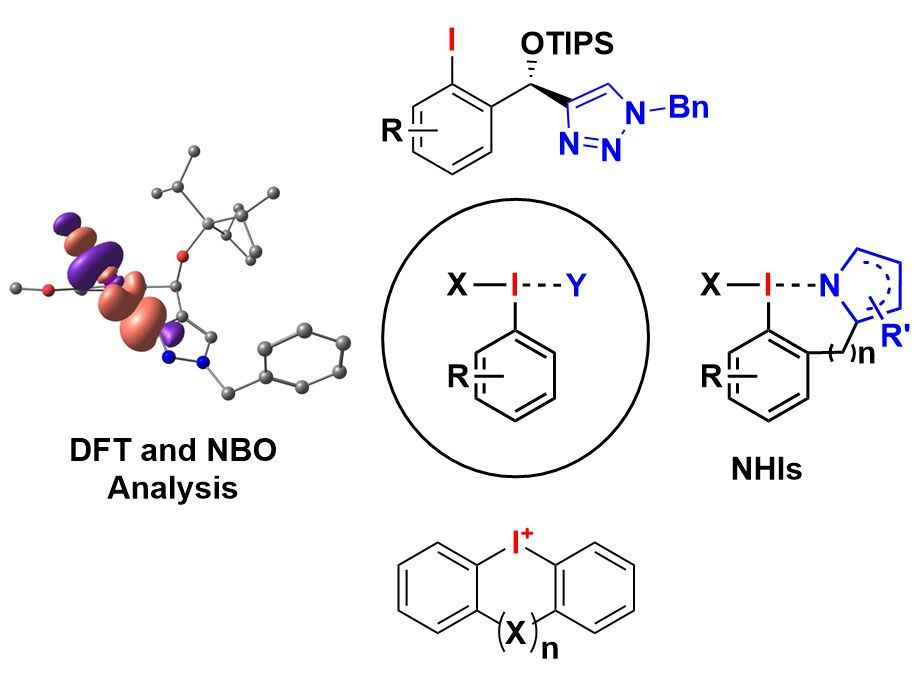
Hypervalent iodine compounds are well-established reagents for oxidation reactions, oxidative couplings and Umpolung strategies. Due to their high stability and convenient reactivity, even under mild reaction conditions, they meanwhile found a variety of applications not only in natural product synthesis but also in materials and medicinal chemistry. In this research field we develop novel hypervalent iodine reagents with so far unseen reactivities. A particular focus is given to the development of (pseudo)cyclic N-heterocylce-stabilzed iodanes (NHIs). As complement reagents to typical O-stabilized hypervalent iodine compounds, NHIs are characterized by a straight-forward and modular synthesis, a high variability, a remarkable reactivities and stabilities. Based on N-heterocycles as stabilizing groups we are also intended to develop novel chiral hypervalent iodine reagents and aryl iodides and apply them in catalytic enantioselective reactions. As another class of highly interesting hypervalent iodine compounds we investigate (a)cyclic diaryliodonium salts. These reagents can be applied for a rapid synthesis of a plethora of carbo- and heterocycles. We develop novel efficient one-pot protocols for their efficient synthesis and apply them as substrates or reagents in organic synthesis. To get a better rational for the observed reactivity we also perform DFT-calculations accompanied by natural bonding orbital (NBO) analysis of our newly synthesized iodanes.
Minimalistic Peptide Assemblies
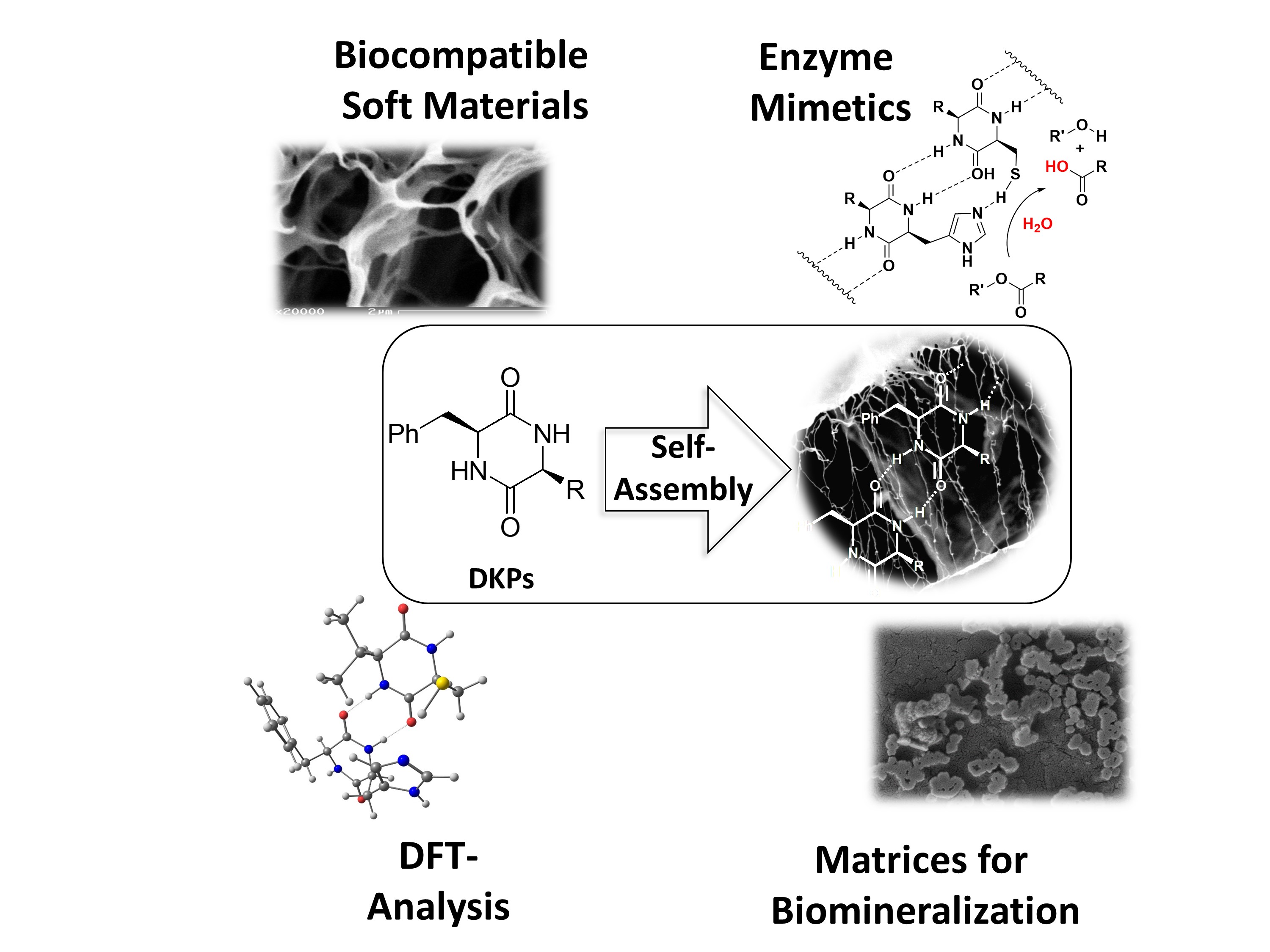
In addition to fundamental oxidative C-X bond forming reactions, we are highly interested in iodine-mediated oxidative domino reaction to construct carbo- and heterocyclic core structures from readily available starting materials. As an example, we recently developed an efficient halocyclization/cycloaddition/ elimination cascade reaction that is truly catalytic in iodine. link
Furthermore, we are interested in iodide-catalyzed oxidative domino reaction for the de novo synthesis of interesting heterocyclic core structures. In this regard, we could recently develop a novel iodide-catalyzed decarboxylative iodination/oxidation/condensation cascade to construct highly substituted oxazoles from aryl ketones and amino acids. link
ESIPT Luminophores
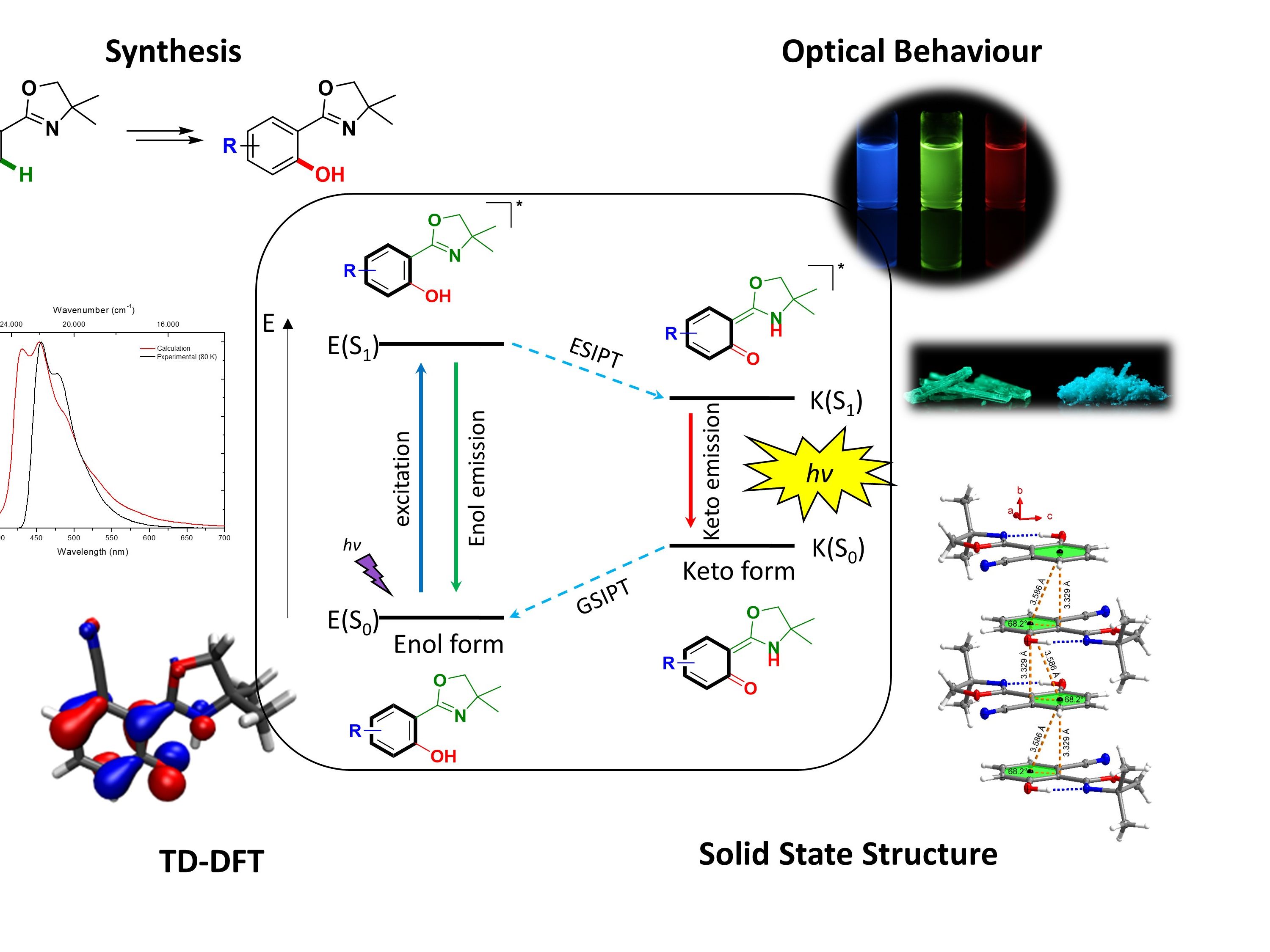
Small high-emissive organic fluorophores have broad applications in material science, in particular as luminophores for OLEDs, in fluorescent sensor or as dyes in bioimaging. They are characterized by a low toxicity and easy synthesis. We develop novel minimalistic fluorophores based on an Excited-State Intramolecular Proton Transfer (ESIPT) with a particular focus on ortho-hydroxy oxazolin-based. We develop novel reaction methods for the efficient synthesis of these novel minimalistic fluorophores and systematically study their optical properties. Through TD-DFT calculations we further try to rationally understand the observed characteristics on a molecular orbital level.
