Research
Selected Research Topics

In the area of molecular switches, we are interested in developing efficient ways of functionalising switchable molecules. Although halogenated azobenzenes typically serve as the electrophilic component in a cross-coupling reaction, it can be advantageous to be able to use them as a nucleophilic cross-coupling partner as well. For this purpose, we developed a palladium catalysed stannylation protocol, which converts iodinated azobenzenes into the corresponding stannanes in high yields. These can then be subjected to Stille cross-coupling conditions, where the products are obtained in excellent yields.
J. Strueben, P. J. Gates, A. Staubitz, J. Org. Chem. 2014, 79, 1719–1728.
A relatively new topic in the area of molecular switches are mechanophoric polymers. We are currently investigating new ways to functionalise the mechanophoric spiropyrans and their uses in materials science.
M. Schulz-Senft, M. Lipfert, A. Staubitz, Chem. Unserer Zeit 2014 , 48, 200–214.
You can find further publications on this topic here.
Our group is interested in the implementation of inorganic main group elements in existing organic molecules.
Recently, the chemistry of 1,2-azaborines, which are benzene analogue in which a C=C bond is replaced with an isoelectric B-N-unit, has become a research focus. This substitution can lead to a higher absorption intensity of light and bathochromic shift compared to a full substitution of all C=C-units with B-N-motifs (borazine), which led to nearly no absorption.[1]
Furthermore, we are investigating a cumulative B-N-substitution effect by incorporation of 1,2-azaborines in polymeric structures. For this purpose, we were able to prepare a B-N-polystyrene using a solvent-free radical polymerization approach.
A first analysis showed that the obtained poly(N-methyl-B-vinyl-1,2-azaborine) possesses a high molecular weight (MW = 24.9 kDa). Moreover, a thorough comparison between the poly(2-methylstryrene) and the poly(N-methyl-B-vinylazaborine) by NMR spectroscopy, TGA, DSC and GPC showed significant differences between these polymers (PDI, Tg, etc.).[2]
In our work, we try to harvest the unique electronic properties of a covalent B-N-bond. B-N-motifs can have a strong influence on structures for their application.
This project of the Staubitz group is financially supported from the Emmy-Noether-foundation.
--
[1] A. M. Daly, C. Tanjaroon, A. J. V. Marwitz, S.-Y. Liu, S. G. Kukolich, J. Am. Chem. Soc. 2010, 132, 5501.
[2] B. Thiedemann, P. J. Gliese, J. Hoffmann, P. G. Lawrence, F. D. Sönnichsen, A. Staubitz, Chem. Comm. 2017, 53, 7258.
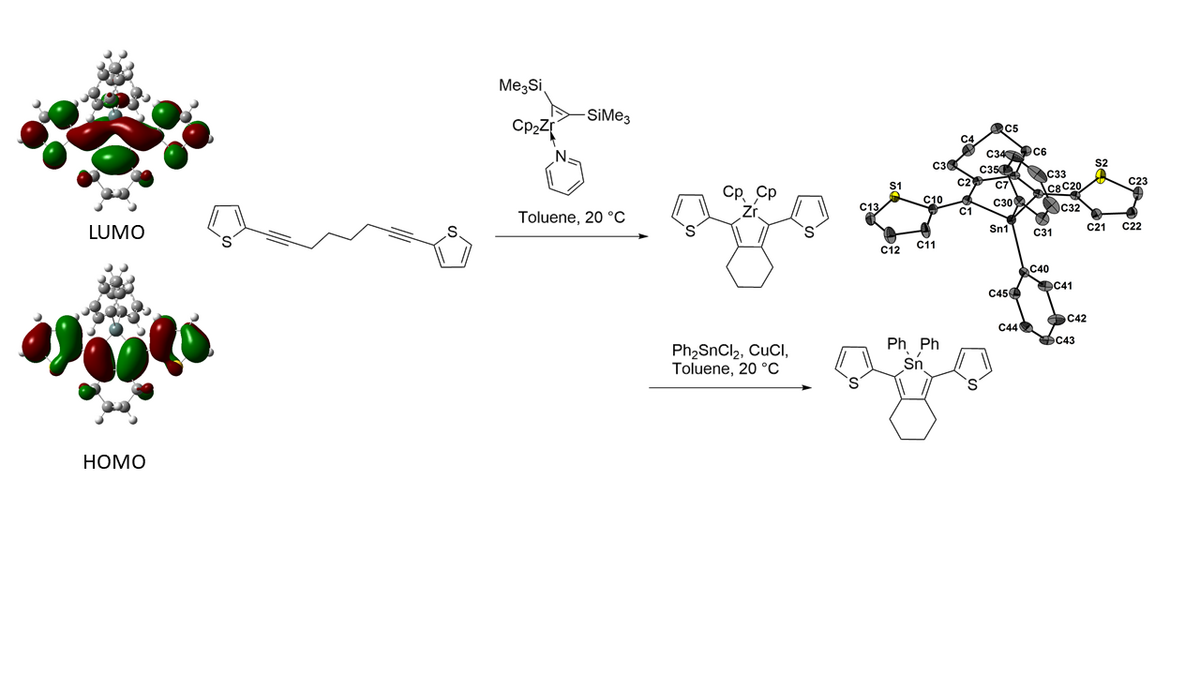
Group 14 metalloles (E = Si, Ge, Sn, Pb) are non-aromatic heterocycles which is in contrast to analogs such as thiophene, furan or pyrrole. However, they exhibit another form of conjugation (σ* π*-conjugation or rather hyperconjugation) instead, which leads to unique (opto)electronic properties. This conjugation is very low in their carbon analog, but replacement of the methylene part by a stannylene (or rather a silylene, germylene or plumbylene) moiety strengthens the conjugation extremely. Due to the efficient interactions of the σ* orbital of the exocyclic E-R bonds and the π* orbital of the diene moiety, ELUMO is remarkable reduced and therefore the HOMO-LUMO gap is narrower in comparison to cyclopentadiene.[1]
Since stannoles have been first synthesized in 1959,[2] different synthetic routes,[3] aromaticity,[4] (opto)electronic properties[1] and reactivity[5] have been investigated.
One powerful tool to generate stannoles is the transmetalation from zirconacyclopentadienes. Such precursor molecules are constructed by C-C coupling of alkynes in the presence of a low-valent zirconocene complex “Cp2Zr”. In a larger study the two most utilized zirconium sources, Negishi´s reagent and Rosenthal´s reagent, have been compared regarding reaction time, conversion and stability of the zirconacyclopentadiene inside the reaction mixture.[6]
These group 14 metalloles show great potential for electronic devices because of their outstanding (opto)electronic properties. However, so far only polymers bearing siloles and germoles have been incorporated.[7] In this regard, about stannoles only little is known, which is why we are interested to explore this research area.[8]
---
[1] I.-M. Ramirez y Medina, M. Rohdenburg, F. Mostaghimi, S. Grabowsky, P. Swiderek, J. Beckmann, J. Hoffmann, V. Dorcet, M. Hissler, A. Staubitz,
Inorg. Chem. 2018, 57, 12562-12575.
[2] F. C. Leavitt, T. A. Manuel, F. Johnson, J. Am. Chem. Soc. 1959, 81, 3163-3164.
[3] a) A. J. Ashe, L. L. Lohr, S. M. Al-Taweel, Organometallics 1991, 10, 2424-2431; b) P. J. Fagan, W. A. Nugent, J. C. Calabrese, J. Am. Chem. Soc.
1994, 116, 1880-1889; c) B. Wrackmeyer, G. Kehr, S. Ali, Inorg. Chim. Acta 1994, 216, 51-55; d) J. Krause, K.-J. Haack, K.-R. Pörschke, B. Gabor, R.
Goddard, C. Pluta, K. Seevogel, J. Am. Chem. Soc. 1996, 118, 804-821; e) G. P. M. van Klink, H. J. R. de Boer, G. Schat, O. S. Akkerman, F.
Bickelhaupt, A. L. Spek, Organometallics 2002, 21, 2119-2135.
[4] M. Saito, M. Shimosawa, M. Yoshioka, K. Ishimura, S. Nagase, Organometallics 2006, 25, 2967-2971.
[5] J. Dubac, A. Laporterie, G. Manuel, Chem. Rev. 1990, 90, 215-263.
[6] S. Urrego-Riveros, I.-M. Ramirez y Medina, D. Duvinage, E. Lork, F. D. Sönnichsen, A. Staubitz, Chemistry – A European Journal 2019, 25,
13318-13328.
[7] a) Z. Li, Y. Q. Dong, J. W. Y. Lam, J. Sun, A. Qin, M. Häußler, Y. P. Dong, H. H. Y. Sung, I. D. Williams, H. S. Kwok, B. Z. Tang, Adv. Funct. Mater. 2009,
19, 905-917; b) D. Gendron, P.-O. Morin, P. Berrouard, N. Allard, B. R. Aïch, C. N. Garon, Y. Tao, M. Leclerc, Macromolecules 2011, 44, 7188-7193.
[8] a) J. Linshoeft, E. J. Baum, A. Hussain, P. J. Gates, C. Näther, A. Staubitz, Angew. Chem. Int. Ed. 2014, 53, 12916-12920; b) W.-M. Zhou, I. Tomita,
Journal of Inorganic and Organometallic Polymers and Materials 2009, 19, 113-117.
Author: Isabel-Maria Ramirez y Medina
Since the ground breaking discovery of semi conducting polymers, by Heeger, MacDiarmid and Shirakawa (Nobel Prize in 2000), this area of macromolecular chemistry has received large interest, both from academia and industry. The major attraction of such materials is that often they can be processed relatively easily, are highly tuneable by chemical modification and are often comparatively cheap.
Applications for conductive polymers are abundant: Electroluminescent polymers, organic light emitting diodes, organic field effect transistors and plastic solar cells are just some examples.
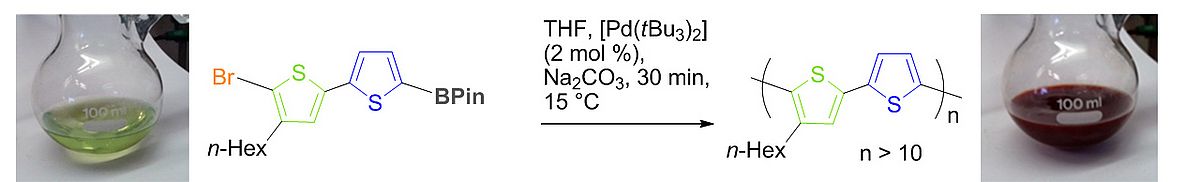
Our group is developing new synthetic methodologies for the preparation of novel hybrid organic-inorganic semiconducting polymers. A very important aspect will also be the control of film morphologies to optimise device performance.
We are interested in accessing new monomers for cross-coupling polymerisation reactions. A very important aspect will also be the control of film morphologies to optimise device performance. Therefore, polymers with controlled regio regularities are required.
Our group is also developing new synthetic methodologies for the preparation of novel hybrid organic-inorganic semiconducting polymers. Incorporation of higher main group elements can e.g. lead to very small band gaps. E.g., we incorporated a stannole heterocycle into the backbone of a semiconducting polymer, the first example of this class of π-conjugated polymers.
You can find further publications on this topic here.
Transition metal catalyzed reactions for the formation of carbon-carbon bonds are among the most important transformations in synthetic chemistry. Especially the functionalization of aromatic heterocycles is receiving increasing interest since these compounds are ubiquitous in pharmaceutical compounds, dyes or advanced organic materials to name but a few.
While cross-coupling reactions which are selective with respect to the electrophilic site are well researched and understood, reactions that differentiate between two different nucleophilic sites, i.e. different metal or hemi-metal functional groups, are exceedingly rare. In fact, only one such reaction where both sites were located at a single aromatic substrate has ever been reported, but no generality has been demonstrated and experimental procedures and data are all but lacking from that communication.
Developing the synthesis of suitable starting materials and generally applicable methods for their selective transformations are therefore most urgently required.
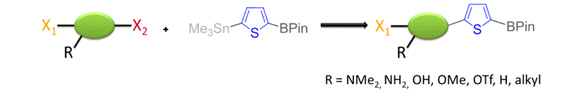
We recently succeeded in developing a new thiophene based dinucleophile, with a stannyl group and a boronic ester. We could show that it was possible to first perform a selective Stille coupling with a variety of electrophiles in very good yields, followed by a Suzuki coupling.
We extended this methodology to a high yielding, both nucleophile and electrophile selective cross-coupling reaction with aromatic rings. The reaction is general with respect to functional groups. Furthermore, the products still contain a boronic ester and a bromide. These two functional groups allow them to be easy-to-prepare, highly complex starting materials for further reactions, avoiding protecting group transformations.
A. C. J. Heinrich, B. Thiedemann, A. Staubitz, Org. Lett. 2013, 15, 4666-4669.
You can find further publications on this topic here.
Equipment
Click here for a list of available devices.
