Salt-induced self-assembly of nanofibrous fibrinogen scaffolds
Research
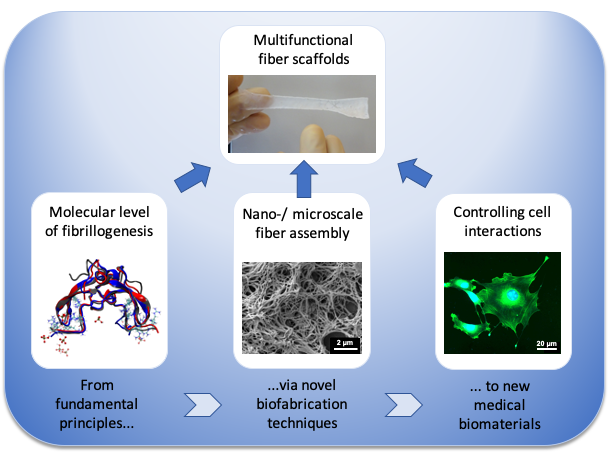
Fibrous biopolymer scaffolds for tissue engineering
Our research focus
Tissues are composed of different cell types, which are surrounded by the extracellular matrix (ECM), a dense network of different protein nanofibers. When an injury occurs in a tissue, a nanofibrous fibrin clot initially closes the wound to facilitate cell growth and tissue repair.
Although nanofibrous biopolymer scaffolds are highly attractive for regenerative medicine it still remains challenging to prepare such scaffolds under physiological in vitro conditions. Therefore, our interdisciplinary research focuses on the multi-scale development and characterization of novel biomimetic fiber scaffolds for tissue engineering.
Our research activities span several length scales from the molecular level of protein fibrillogenesis via the nano- and microscale of scaffold assembly towards the development of macroscopic biomaterials.
In vitro fiber assembly
Key techniques
Based on biophysical studies on fiber formation our group develops new biofabrication techniques for the preparation of fibrous scaffolds under physiological in vitro conditions.
Key techniques to fabricate biopolymer fibers that have been developed in our group are:
- Salt-induced self-assembly of fibrinogen into nanofibers
- Template-assisted extrusion of proteins through ceramic nanopores
We also use established methods to develop fibrous biomaterials with new compositions and tailored functionality:
- Self-assembly of nanofibrous collagen scaffolds
- Wet-spinning of polysaccharide microfibers, e.g. chitosan
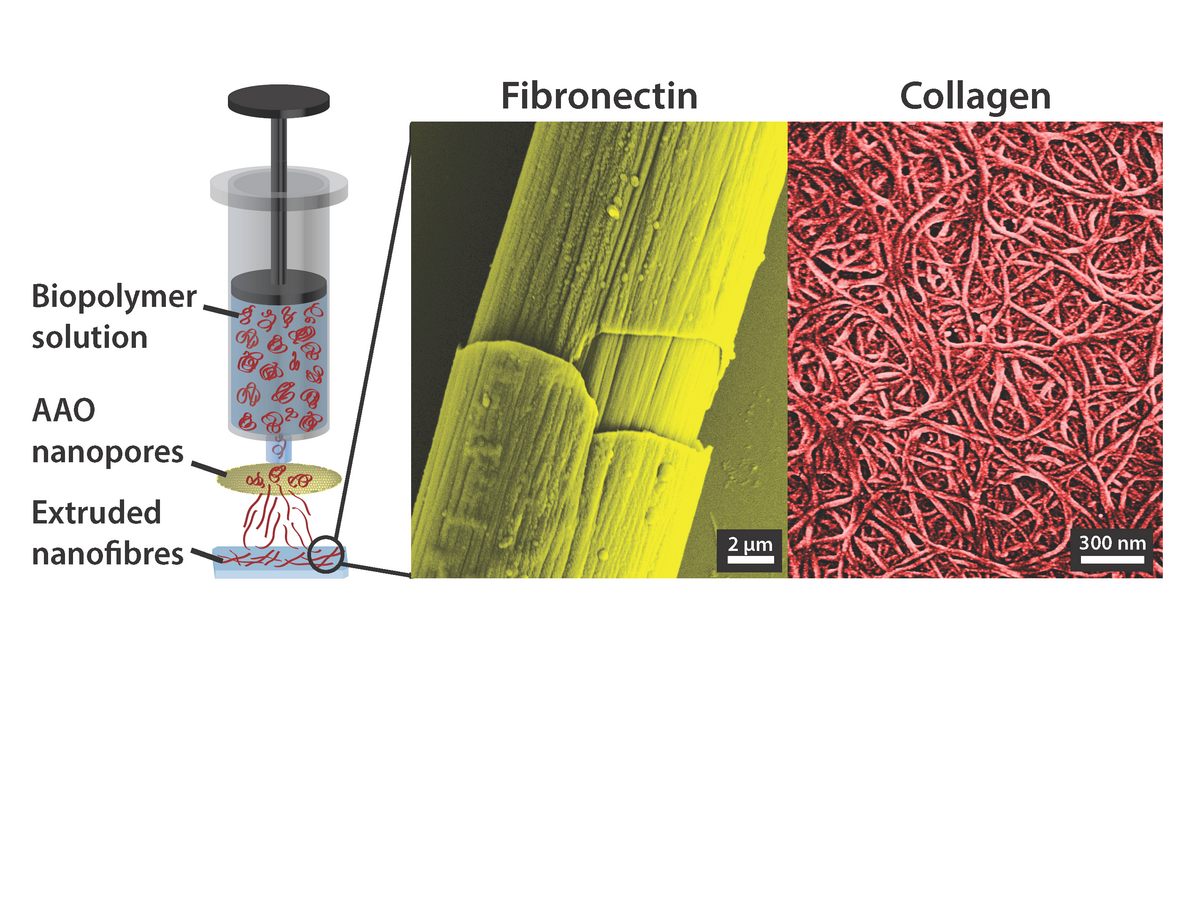
Extrusion of proteins
Template-assisted extrusion of proteins through nanoporous ceramics (AAO) yields different hierarchical arrangements of protein nanofibers (Raoufi et al., Integr Biol 8, 2016).
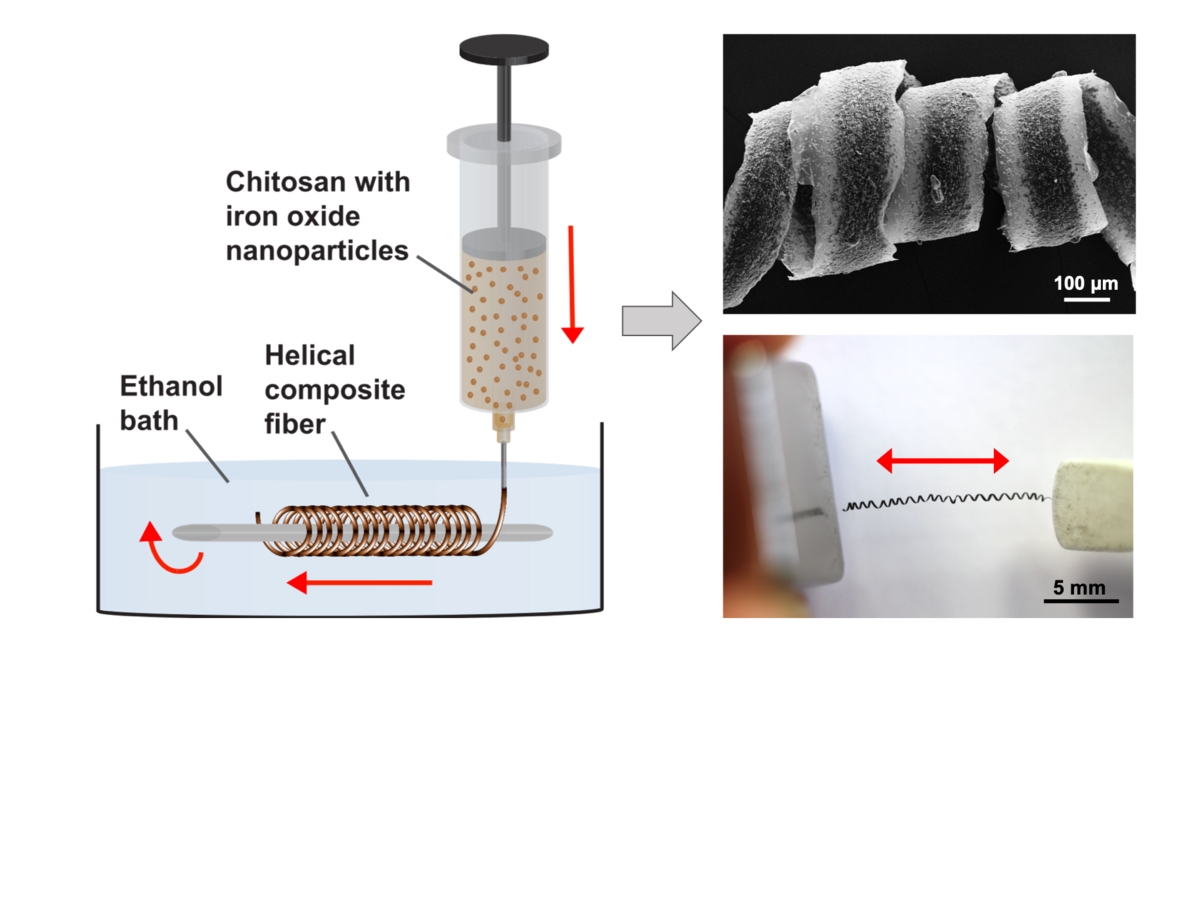
Wet-spinning of chitosan
Wet-spinning of chitosan with embedded magnetic nanoparticles yields helical microfibers for magnetic actuation (Brüggemann, et al., BJNANO 11, 2020).
Molecular level
What are the driving forces during in vitro fibrillogenesis?
Changes on the molecular level of a biomaterial can influence its biofunctionality on different length scales. These structural changes can affect molecular signaling and the interaction with cells. For the development of future biomaterials, it is therefore important to understand the molecular mechanisms that occur during fiber assembly.
Our goal is to establish fibrous protein scaffolds as a biophysical model system for protein fibrillogenesis in a cell-free environment. To analyze conformational changes in fibrous protein scaffolds we use various modern characterization methods including Förster resonance energy transfer (FRET) measurements, circular dichroism (CD) and Fourier transform infrared (FTIR) spectroscopy.

FRET analysis
FRET analysis of extruded fibronectin fibers reveals lasting changes in the protein conformation (Raoufi et al., Nano Lett 15, 2015).
Nano-/microscale
How can the nanoarchitecture and functionality of fibrous biomaterials be tailored?
In native tissue, a complex interplay of topographical, biochemical and mechanical cues governs molecular and cellular interactions. Hence, controlling the nano-/microarchitecture and biofunctionality of a biomaterial is important to achieve tailored interactions during wound healing and tissue repair.
We use state-of-the-art microscopy techniques to characterize our fibrous biomaterials from the nano- to the microscale including scanning electron, atomic force and confocal microscopy. Towards future applications in tissue engineering we also characterize the stability and degradation properties of fibrous biopolymer scaffolds under varying environmental conditions including temperature changes or enzyme activity.
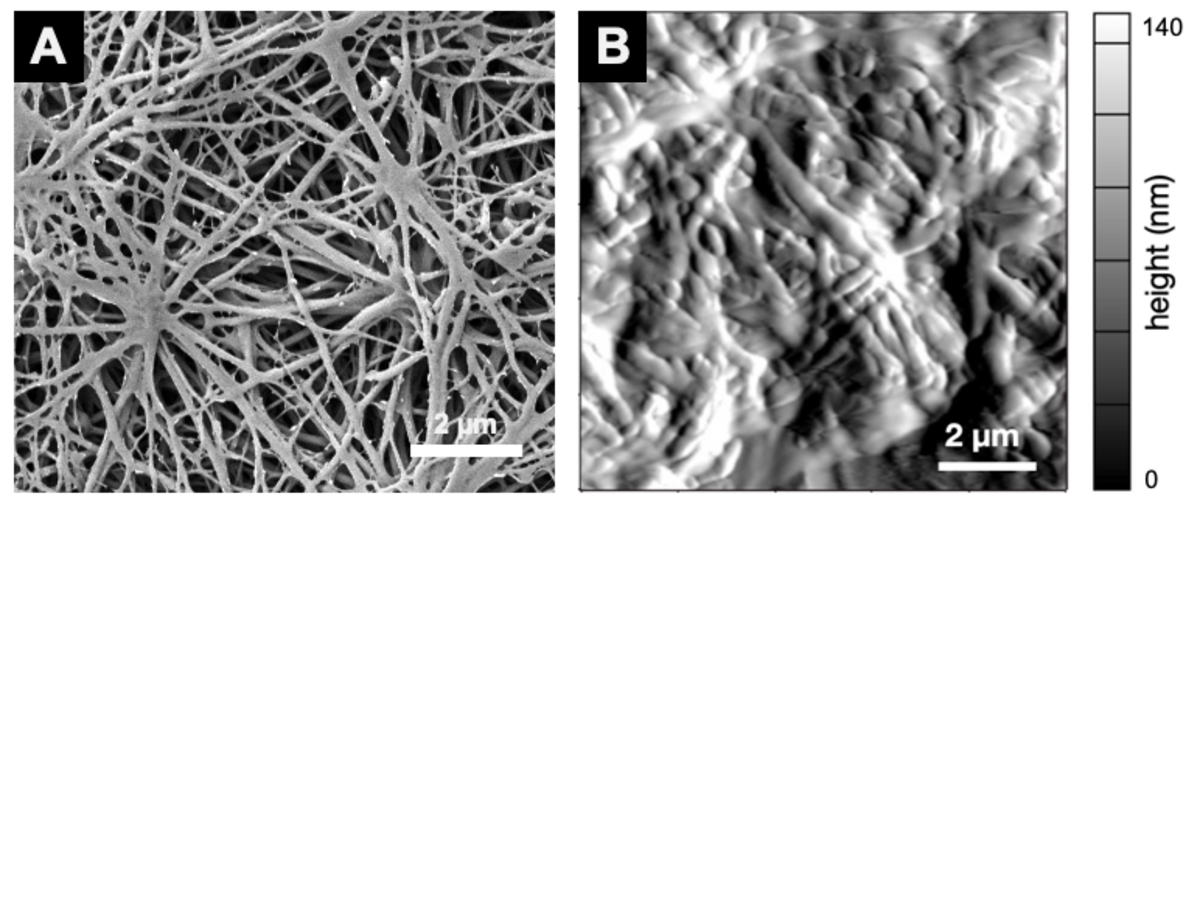
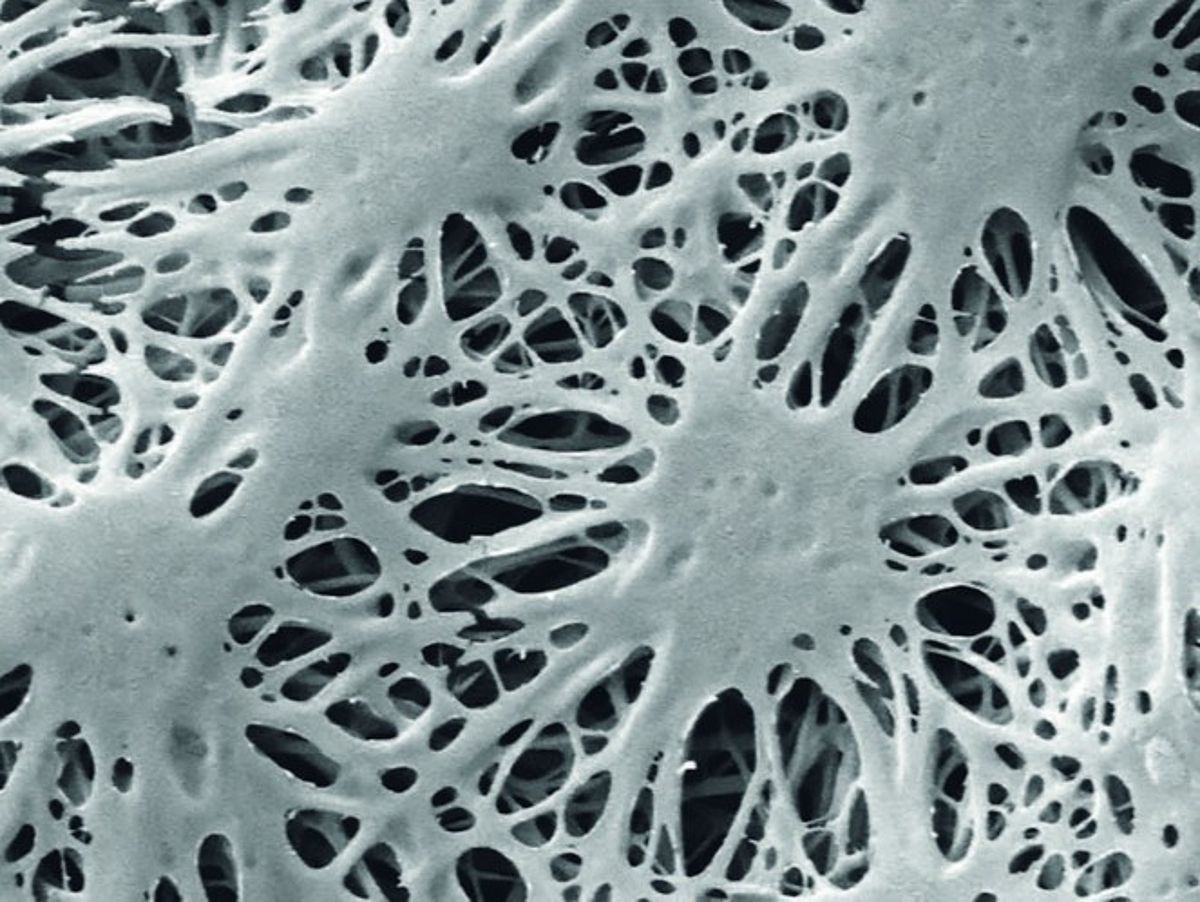
Fibrillogenesis of fibrinogen
Salt-induced self-assembly induces fibrillogenesis of fibrinogen under physiological buffer conditions. (A) SEM image of dried fibrinogen nanofibers shows a highly porous scaffold nanoarchitecture (Stapelfeldt et al., Biofabrication 11, 2019), (B) AFM image of fibrinogen nanofibers in aqueous environment (Stapelfeldt et al., Nano Lett 19, 2019).
Cell interaction
How do cells interact with fibrous biomaterials?
The complex interplay of cell-biomaterial interactions is one of the most important challenges in tissue engineering. Towards future applications in regenerative medicine we want to understand the interaction of our new fibrous biomaterials with different cell types, such as fibroblasts or keratinocytes.
We study how cells proliferate and migrate on fibrous biomaterials and how the actomyosin cytoskeleton and cell morphology are changed. Moreover, we are interested in the interaction of fiber scaffolds with bacterial cultures to screen them for antimicrobial properties. Typical techniques we use to study cell interactions are viability assays, fluorescence microscopy or live-cell tracking in combination with scanning electron microscopy.
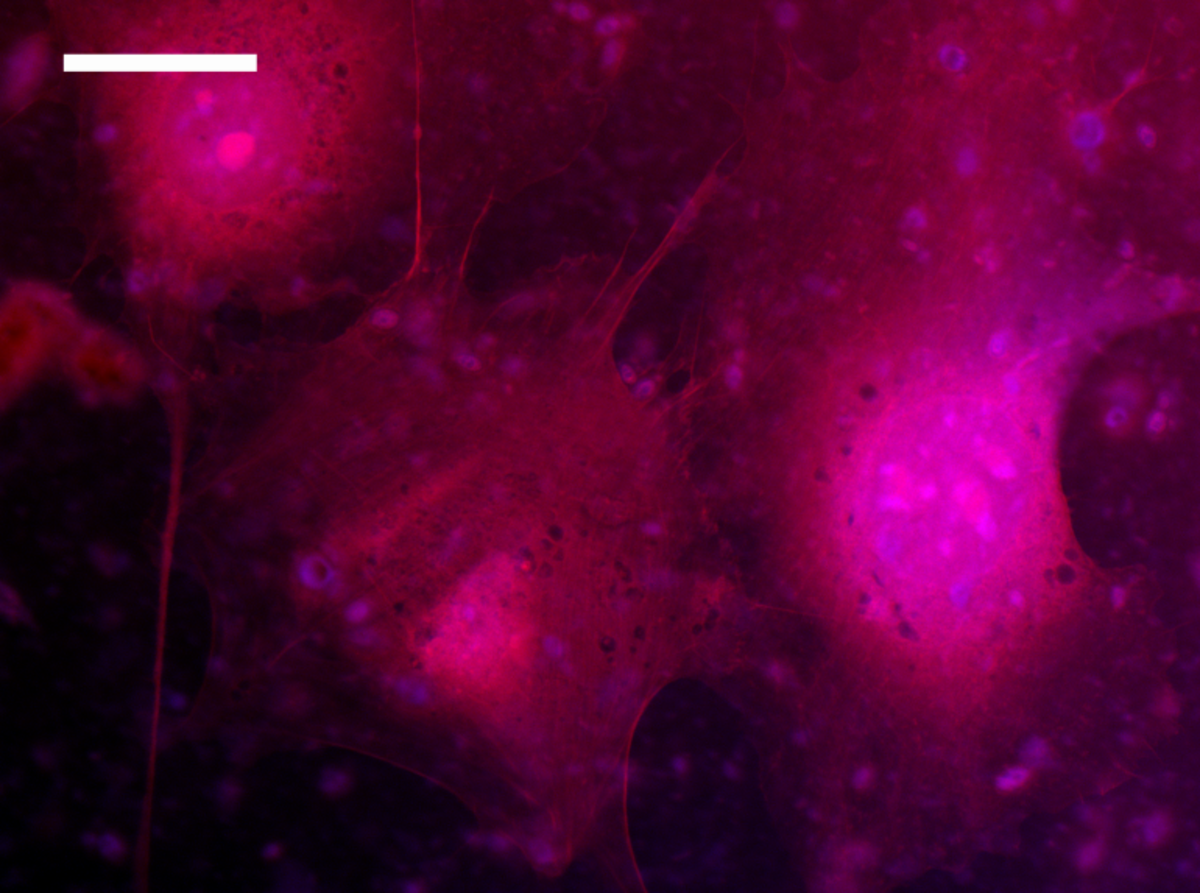
Fibroblasts on collagen nanofibers
Fluorescence microscopy image of fibroblasts on collagen scaffolds, scale bar: 20 µm (Suter et al., Biofabrication 13, 2020)
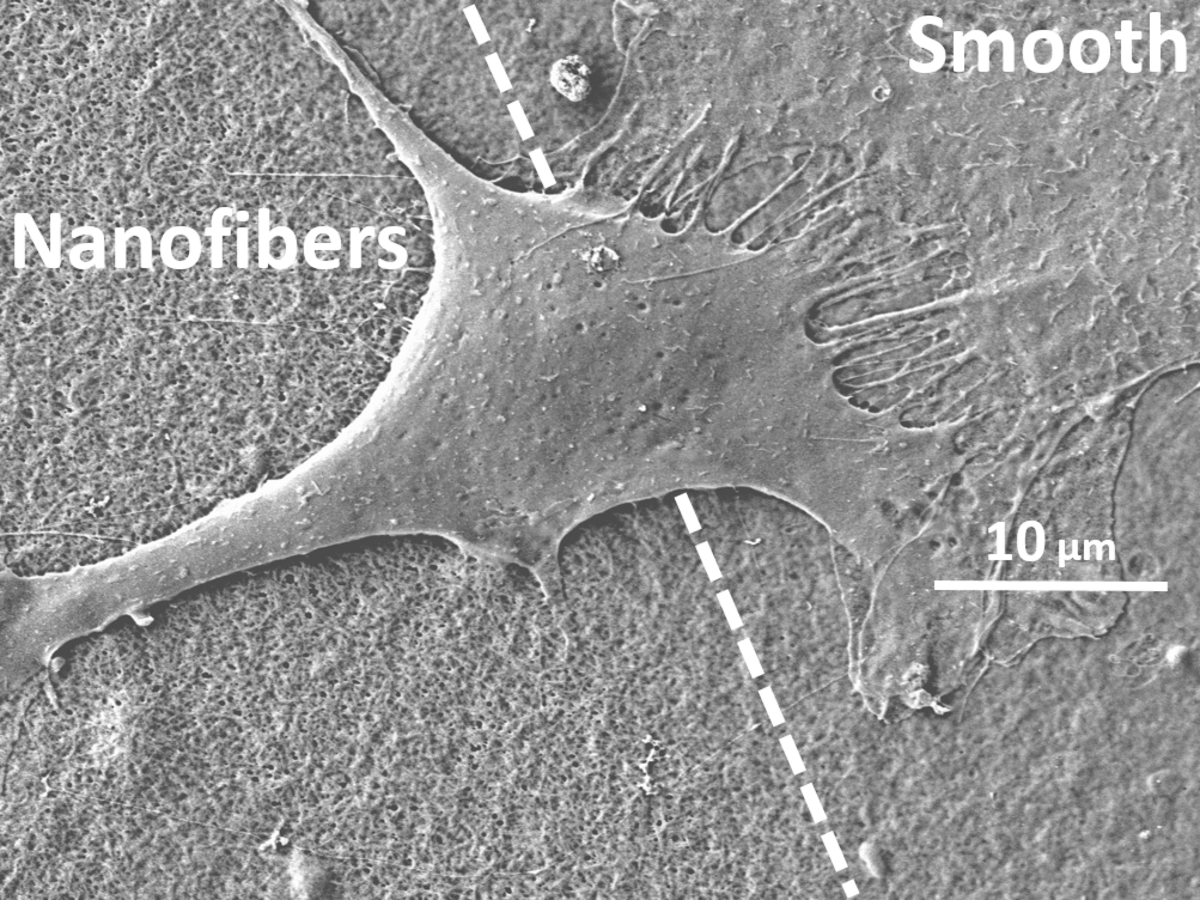
Fibroblast on collagen scaffolds
Scanning electron microscopy image of a fibroblast on collagen with fibrous and smooth topography (Suter et al., Biofabrication 13, 2020)
Macroscopic scaffolds
How can fibrous biopolymer scaffolds be scaled up?
To identify future strategies that efficiently treat large tissue defects, the scalability of a biofabrication processes is very important. We therefore aim to upscale our new biofabrication routines for future tissue engineering applications, for example by introducing polymer molding.
To facilitate optimum tissue regeneration the mechanical properties of macroscopic biomaterials need to be tailored. Hence, we also aim at optimizing the tensile and compression properties of our fibrous biomaterials for tissue repair.
Moreover, we want to understand how cells react to changes in the nanotopography in real time. Therefore, we combine protein self-assembly with polymer printing to prepare protein scaffolds with spatially controlled surface topographies to study their interaction with cells

Detachment of nanofibrous fibrinogen scaffolds
Controlled detachment of nanofibrous fibrinogen scaffolds can be achieved by a surface modification of the underlying substrate and a crosslinking step (Stapelfeldt et al., Biofabrication 11, 2019).

Macroscopic fibronogen scaffolds
Macroscopic scaffolds of nanofibrous fibrinogen can be prepared by salt-induced self-assembly with dimensions up to 10 cm in length.
Nanoporous protein-ceramic composites
How do inorganic nanomaterials interact with proteins and cells?
When inorganic nanomaterials are used as implant material, they are immediately coated with proteins from blood and other body fluids. Therefore, we also want to understand the interaction of inorganic nanomaterials, like porous ceramics, with proteins and cells.
In cooperation with the MIMENIMA research training group at the University of Bremen we study the interaction of different proteins involved in cell adhesion and blood coagulation with porous ceramics like anodized aluminum oxide (AAO). To improve cell growth, we develop different surface functionalization strategies for ceramic nanomaterials using our established fibrous protein scaffolds.
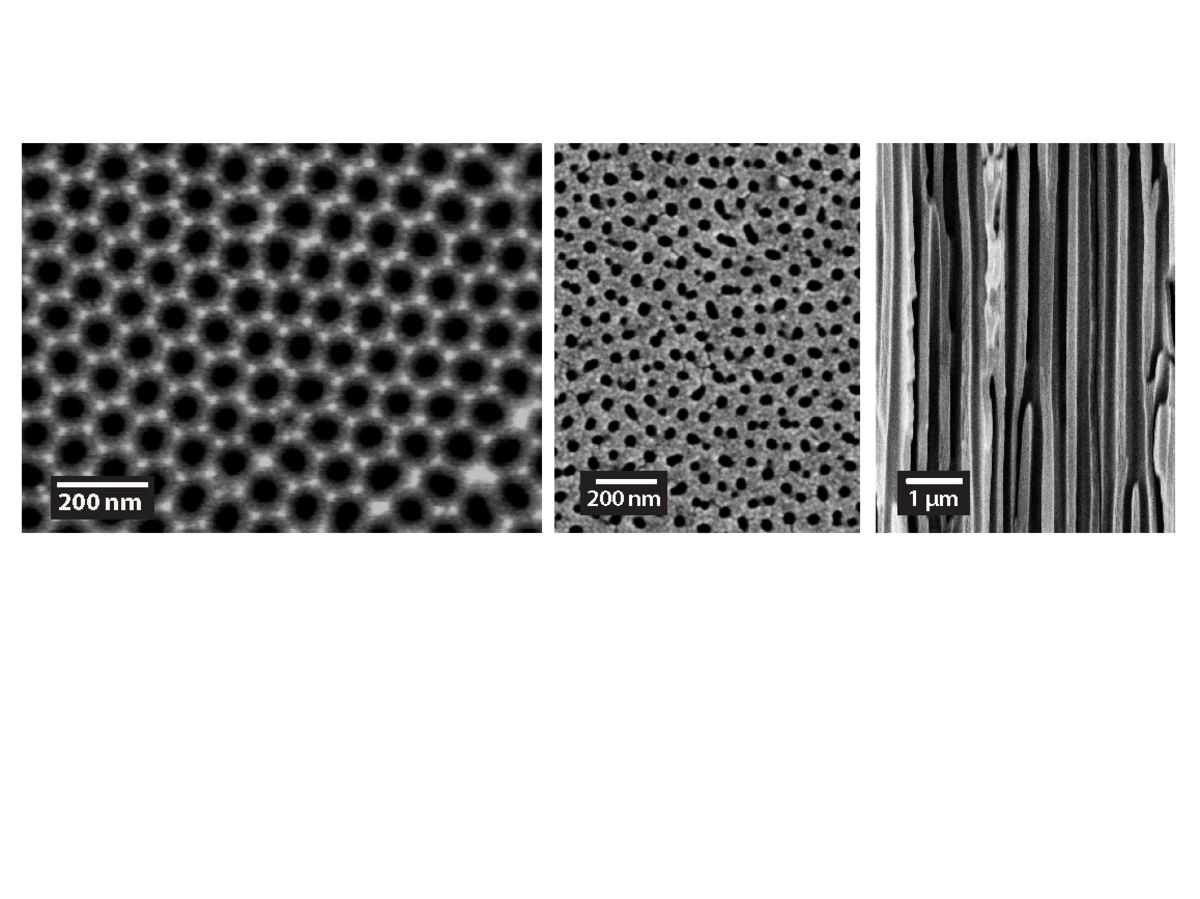
AAO nanopores
Anodized aluminum oxide (AAO) nanopores are prepared by an electrochemical etching process, which results in the self-assembly of vertical nanopores with well-controllable geometries.