Elisabete Trindade Pedrosa, Cornelius Fischer, Luiz F. G. Morales, Ricarda D. Rohlfs, Andreas Luttge
Chemical Geology (2021) 559, 119952
http://doi.org/10.1016/j.chemgeo.2020.119952
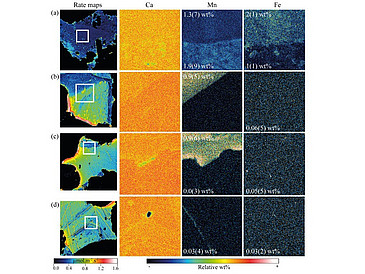
Chemical zoning of crystals is often found in nature. Crystal zoning can play a role in a mineral's thermodynamic stability and in its kinetic response in the presence of fluids. Dissolution experiments at far-from-equilibrium conditions were performed using a sandstone sample containing calcite cement crystal patches. The surface normal retreat of the calcite crystals was obtained by vertical scanning interferometry (VSI) in their natural position in the rock. Dissolution rate maps showed contrasting surface dissolution areas within the crystals, in the same locations where electron microprobe (EMP) maps showed the presence of manganese (Mn) and iron (Fe) substitutions for calcium in the calcite structure. Iron zoning was only identified in combination with manganese. Maximum registered manganese contents were 1.9(9) wt% and iron were 2(1) wt%. Manganese zoning of only 0.9(5) wt% resulted in around 40% lower dissolution rates than the adjacent pure calcite zones. The combination of both Mn and Fe cation substitutions resulted in one order of magnitude lower dissolution rates compared to pure calcite in the same sample. These results show that mineral zoning can significantly affect reaction rates, a parameter that needs better understanding for the improvement of kinetic geochemical models at the pore scale.