Thorsten M. Gesing, M. Mangir Murshed, Selina Schuh, Oliver Thüringer, Konrad Krämer, Tim Neudecker, Cecilia B. Mendive & Lars Robben
Journal of Materials Science 57 (2022): 19280-19299
https://doi.org/10.1007/s10853-022-07854-w
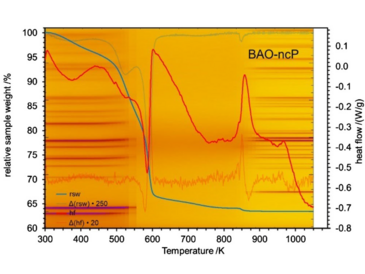
A new precursor for the formation of mullite-type visible-light active photocatalyst Bi2Al4O9 has been identified. The crystal structure of the organic–inorganic hybrid perovskite can be described using the hexagonal setting of the rhombohedral unit cell with lattice parameters a = 1.1342(2) nm, c = 2.746(1) nm, and V = 3.059(2) nm3. The presence of di-nitro-glycerin as organic component, which is centered together with two bismuth atoms at the A-sites of the ABX3-type perovskite, suggests for doubling of the a- and c-lattice parameters compared to isostructural BiAlO3 perovskite. The nano-crystalline precursor with the chemical composition [Bi2(C3H5N2O7)]Al4[O9(□1-x(H2O)x)3] (□: vacancies) decomposes at 540(10) K to a quantum-crystalline phase with an average crystallite size of 1.4(1) nm, refined from X-ray powder data Bragg reflections and confirmed by atomic pair distribution function data analysis. Further heating enables a controlled formation of quantum- or nano-crystalline mullite-type phases, depending on temperature and time. The same precursor structure could also be obtained as iron-containing phase and for Al/Fe solid-solution samples. UV/Vis diffuse reflectance spectroscopy suggests an indirect band-gap transition energy of 3.50(3) eV calculated by the Reflectance-Absorption-Tauc-DASF (RATD) method. Temperature-dependent UV/Vis allows to follow the change of band-gap energy across all associated phase transformations. The long- and short-range appearance of each phase has been presented using X-ray Bragg scattering and total scattering data analyses. This is supported by Raman and infrared spectroscopic investigations complemented by density functional theory (DFT) calculations. Moreover, the theoretical calculation confirms the incorporated di-nitro-glycerin. Thermal stabilities of the phases are investigated by using thermal analysis and temperature-dependent X-ray diffraction.