Bernhard von Boehn, Christopher Penschke, Xiaoke Li, Joachim Paier, Joachim Sauer, Jon-Olaf Krisponeit, Jan Ingo Flege, Jens Falta, Helder Marchetto, Torsten Franz, Gerhard Lilienkamp, Ronald Imbihl
Journal of Catalysis (2020), 385, 255-264
https://doi.org/10.1016/j.jcat.2020.03.016
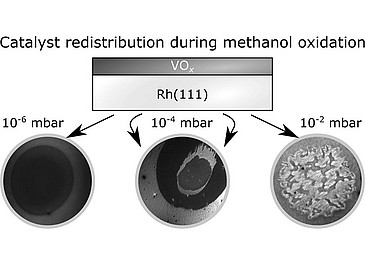
Recent advances in in situ microscopy allow to follow the reaction dynamics during a catalytic surface reaction from ultra-high vacuum to 0.1 mbar, thus bridging a large part of the pressure gap. Submonolayer vanadium oxide films on Rh(1 1 1) have been studied during catalytic methanol oxidation in situ with spatially resolving imaging techniques. At 10−6–10−4 mbar VOx condenses into macroscopic circular islands that exhibit a substructure, consisting of a reduced island core and an oxidized outer ring. This substructure arises due to an oxygen gradient inside the VOx islands, which results in different coexisting 2D-phases of VOx on Rh(1 1 1). This substructure is also responsible for a “breathing-like” oscillatory expansion and contraction that the islands undergo under stationary conditions. Using density functional theory, the 2D-phase diagram of VOx on Rh(1 1 1) has been computed. The oscillatory behavior can be understood as a periodic phase transition between two 2D phases of VOx. With a newly developed near ambient pressure – low-energy electron microscope, it was shown that VOx islands disintegrate at 10−2 mbar, resulting in turbulent dynamics.