Metal Oxide Modeling
Modeling Electrochemical Oxide Film Growth
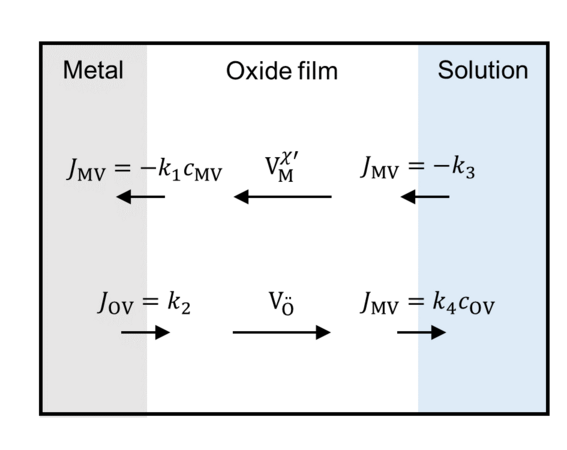
Oxide film growth can be modeled by interfacial reactions and transport of crystal defects through the film. The reaction rate of the interfacial reactions depends on the potential drop at the metal/film and metal/surrounding interfaces. The course of the potential inside the film is given by the charge densities, which can be calculated by the defect concentrations and the concentrations of electrocns and holes.
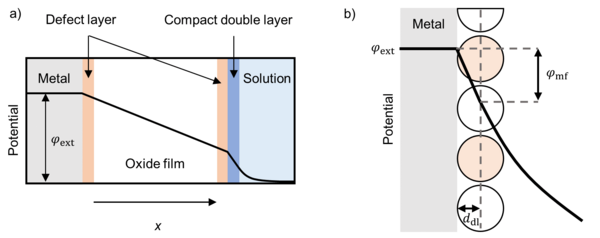
At the interfaces between the metal and the film, and the film and the surrounding are defect layers over which the potential drops linearly. The potential drops at this interfaces determine the reaction rates of the electrochemical reactions. The potential distribution inside the film determines the transport of the charged species (crystal defects, electrons, holes).
This research project is on modeling electrochemical oxide film growth at metal electrodes. Among others, oxide films play an important role in terms of corrosion and in the semiconductor industries. Modeling of oxide films can give deeper insider and understanding of the processes at the interfaces between metal and oxide film and oxide film and surrounding. Thus, corrosion protection can be enhanced and optimized.
Literature
Ingmar Bösing. "Modeling electrochemical oxide film growth—passive and transpassive behavior of iron electrodes in halide-free solution." npj Materials Degradation 7.1 (2023): 53.
Ingmar Bösing, Jorg Thöming, and Fabio La Mantia. "Modeling of electrochemical oxide film growth–impact of band-to-band tunneling." Electrochimica Acta 406 (2022): 139848.

