Spectroscopy
Spectroscopy
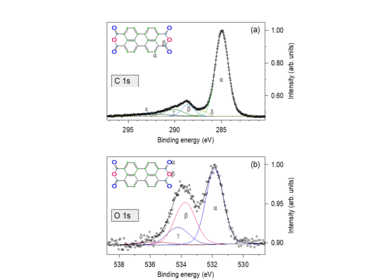
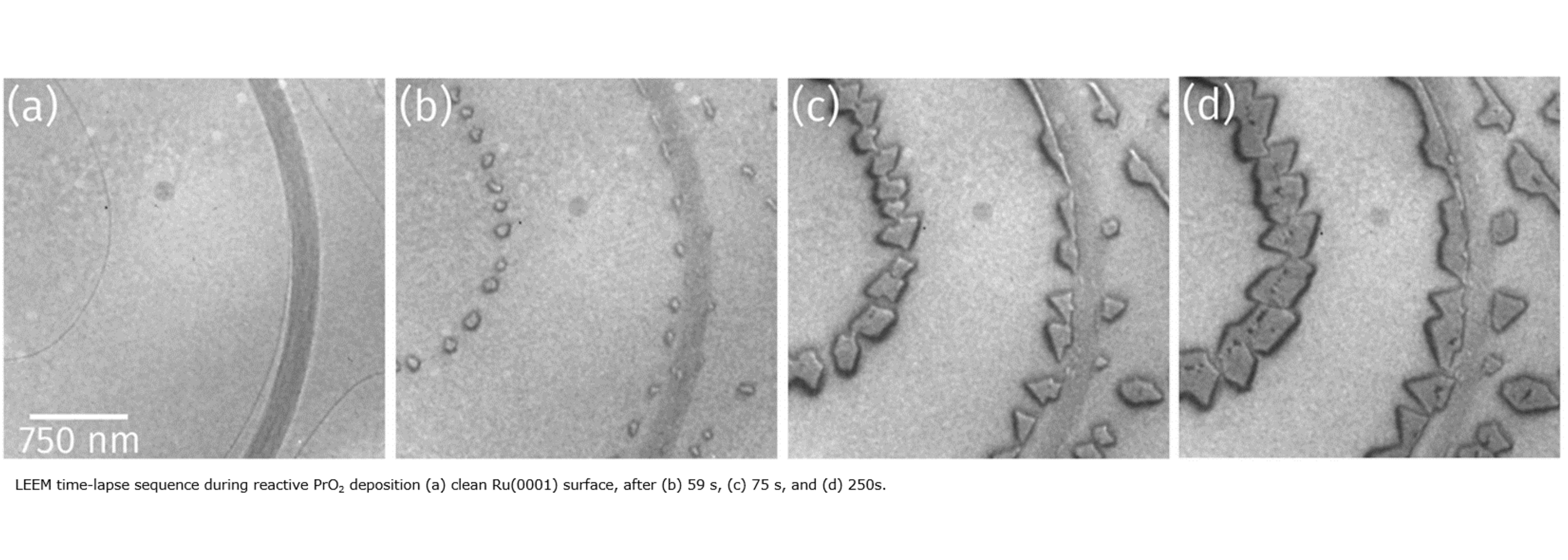
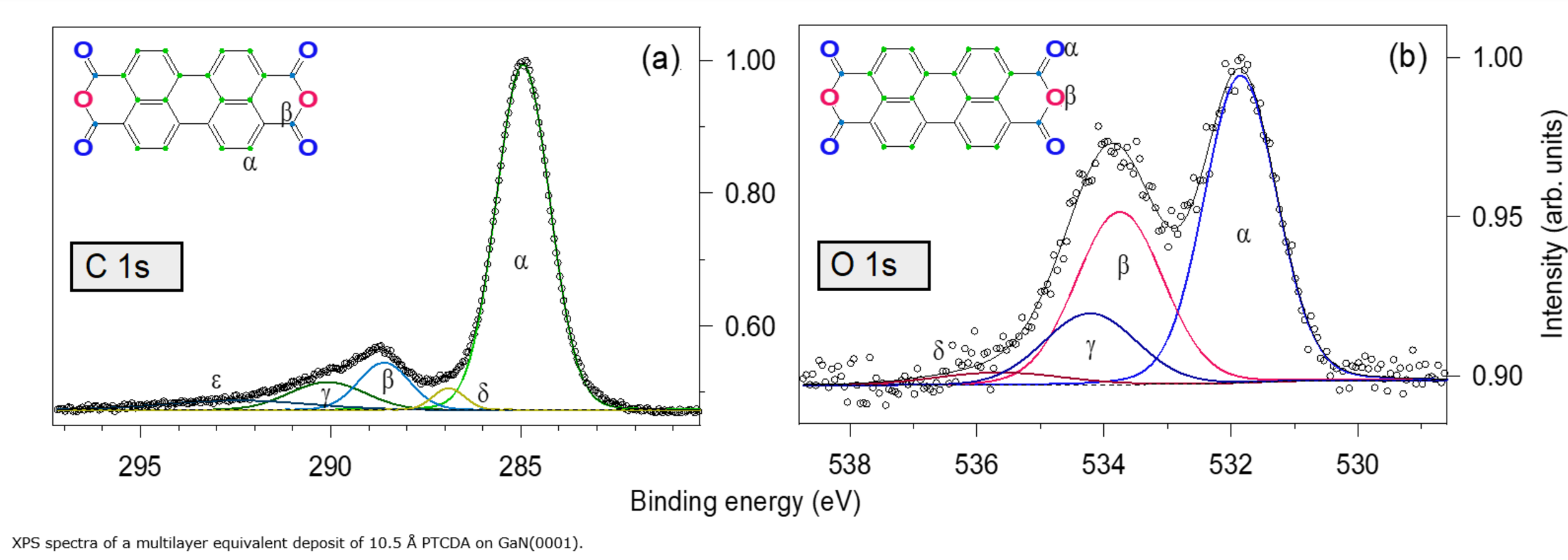
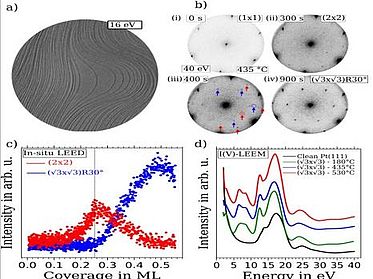
The kinetic energy of electrons emitted from atomic core levels after x-ray photo absorption is characteristic of the respective element and its chemical state, giving rise to individual spectral lines which can be used, e.g., to distinguish different oxidation states with XPS. Combined chemical and structural information are contained in so-called LEEM-IV measurements, where, in a separate experimental setup, low-energy electron reflectivity is recorded as a function of the incident electron energy. Such chemical and structural surface phases can be identified with a lateral resolution of a few tens of micrometers.
Complementary, the vibrational and structural properties of samples can be investigated using Raman and circular dichroism spectroscopy setups.
What kind of result do I get?
With XPS and LEEM both the chemical composition as well as the local structure and morphology of samples can be addressed. Since both techniques employ electrons with rather low energy, the information depth is restricted to a few atomic layers, and a decent electrical conductivity of the samples is required as well as ultra-high vacuum compatibility. Spectra and images can also be taken at elevated temperatures as well as during gas dosing (up to 10-4mbar) to study changes of the chemistry, structure, and morphology during annealing or interaction with gases.
The local bonding geometry can be revealed with vibrational (Raman) spectroscopy. Circular dichroism spectroscopy (CDS) allows for the investigation of chiral structures, e.g. biomolecules adsorbed at interfaces. Both methods, Raman and CDS, are less surface sensitive than the electron-based methods of XPS and LEEM, and can be obtained from a wider class of specimens (e.g. insulators, non-UHV compatible materials.)
Area responsible
Prof Dr Jens Falta
Application Scientists
Dr. Jon-Olaf Krisponeit
University of Bremen
Institute of Solid State Physics
Otto-Hahn-Allee 1, D-28359 Bremen
E-mail: krisponeitprotect me ?!ifp.uni-bremenprotect me ?!.de
Dr. M. Mangir Murshed
University of Bremen
Institute of Inorganic Chemistry and Crystallography
Leobener Straße 7, D-28359 Bremen
E-mail: murshedprotect me ?!uni-bremenprotect me ?!.de
Our key instruments
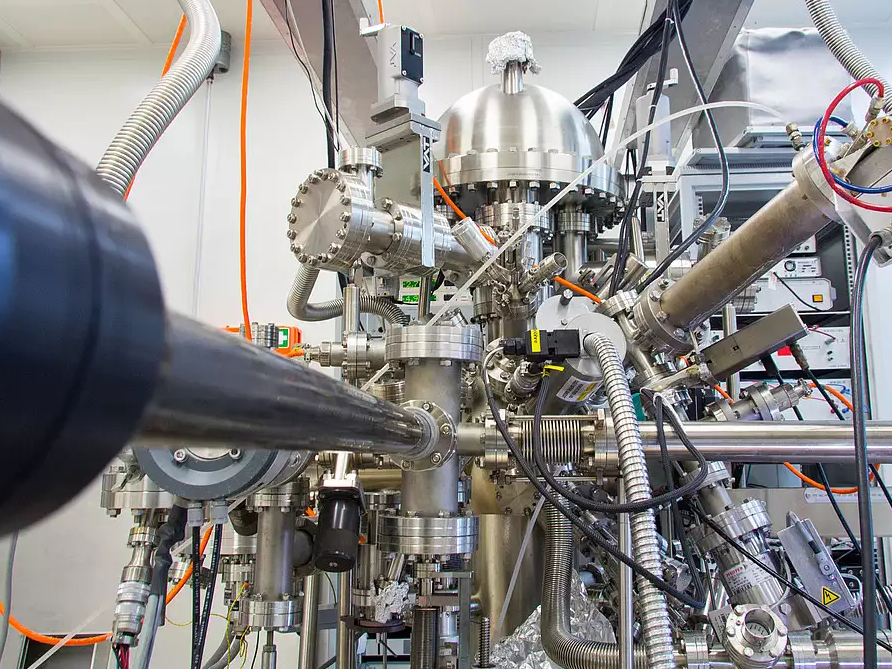
Omicron DAR 400 + EA 125
X-ray photoemission spectrometer
- Twin Al/Mg anode, 125 mm hemispherical analyzer, 7-channel parallel detection unit
- UHV system additionally equipped with low-energy electron diffraction (LEED), scanning tunneling microscope (STM), and evaporation ports
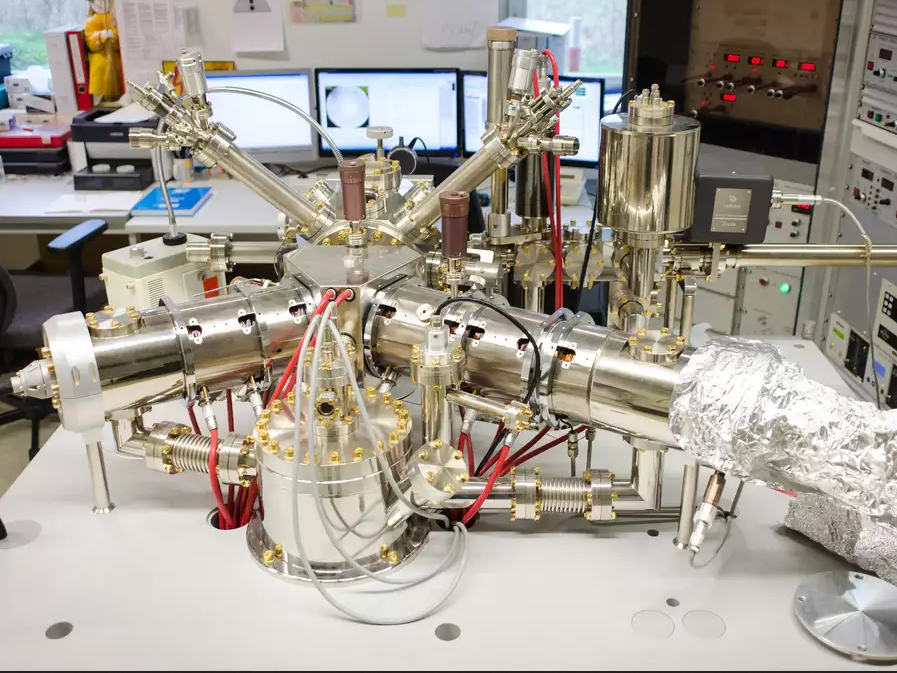
SPE-LEEM Elmitec
Spectroscopic low-energy electron microscope
- Full-field electron microscope with lateral resolution down to 10 nm; diffraction mode for micro-LEED images from selected areas with down to 250 nm in diameter
- Hemispherical energy analyzer
- Video rate imaging for real-time investigations, e.g., during material deposition or gas dosing; 100-1800 K temperature range

Horiba LabRAM Aramis
Raman Spectrometer
- 532 nm, 633 nm and 785 nm lasers
- Measurements possible between 78 K and 1773 K

Applied Photophysics Chirascan
Circular dichroism spectrometer
- Surface and interface characterization
- (Secondary) structure detection of biomolecules
More available instruments
More information about the instrumentation available at MAPEX and MAPEX-CF can be found in the Instrument Database of the MAPEX Center for Materials and Processes.
Research Highlights


Partial-to-fully oxidized spectrum of Ti₃C₂Tₓ MXene-derived TiO₂ free-standing films for nonvolatile high endurance memristive data storage
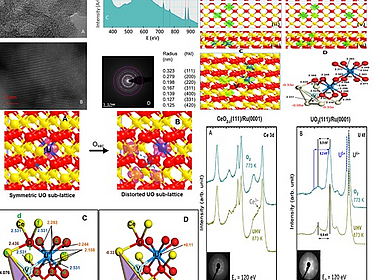
Stabilization of Ce³⁺ cations via U–Ce charge transfer in mixed oxides: consequences on the thermochemical water splitting to hydrogen

Towards coupling agent-free composites made from regenerated cellulose/HDPE by UV radiation-induced cross-linking
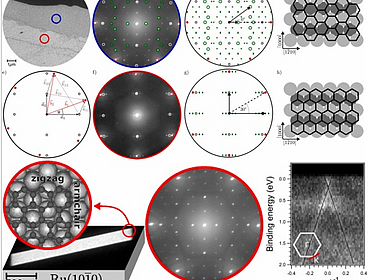
Hexagons on rectangles: Epitaxial graphene on Ru(1010)
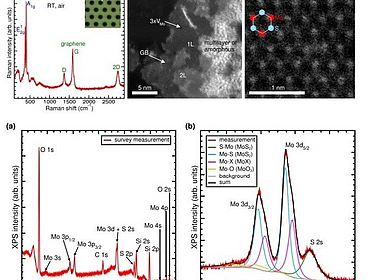
Atomic vs. sub-atomic layer deposition: impact of growth rate on the optical and structural properties of MoS₂ and WS₂

Growth, catalysis, and faceting of α-Ga₂O₃ and α-(InₓGa₁₋ₓ)₂O₃ on m-plane α-Al₂O₃ by molecular beam epitaxy

Gold Nanoparticle-Coated Bioceramics for Plasmonically Enhanced Molecule Detection via Surface-Enhanced Raman Scattering

Growth Mechanism of Single-Domain Monolayer MoS₂ Nanosheets on Au(111) Revealed by In Situ Microscopy: Implications for Optoelectronics Applications
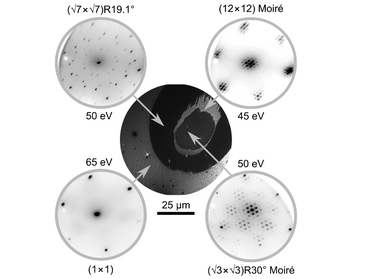
Phase Separation within Vanadium Oxide Islands under Reaction Conditions: Methanol Oxidation at Vanadium Oxide Films on Rh(111)
Plasmonic porous ceramics based on zirconia-toughened alumina functionalized with silver nanoparticles for surface-enhanced Raman scattering

Structural, vibrational, thermal, and magnetic properties of mullite-type NdMnTiO₅ ceramic
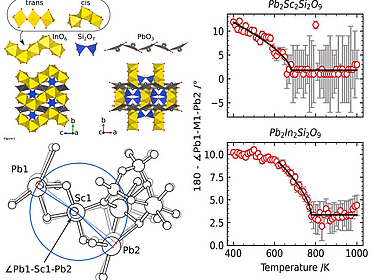
Thermal anomalies and phase transitions in Pb₂Sc₂Si₂O₉ and Pb₂In₂Si₂O₉
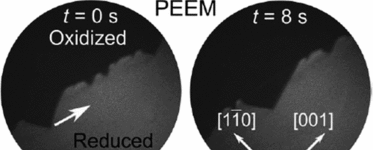
Structural Transitions Driving Interface Pulses in Methanol Oxidation on Rh(110) and VOₓ/Rh(110): A LEEM Study
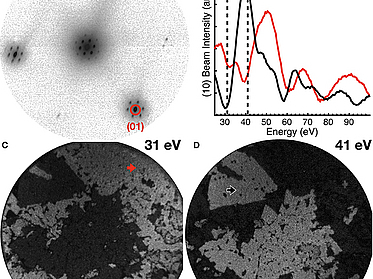
The Transition From MoS₂ Single-Layer to Bilayer Growth on the Au(111) Surface

The morphology of VO₂/TiO₂(001): terraces, facets, and cracks
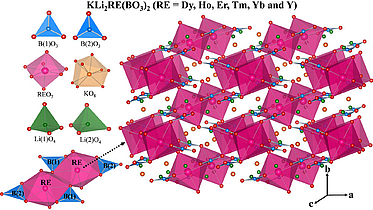
KLi₂RE(BO₃)₂ (RE = Dy, Ho, Er, Tm, Yb, and Y): Structural, Spectroscopic, And Thermogravimetric Studies on a Series of Mixed-Alkali Rare-Earth Orthoborates
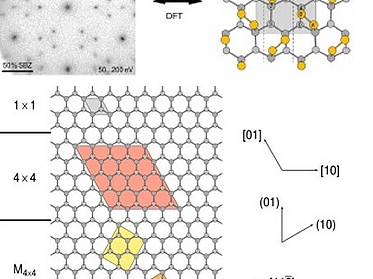
Adsorption of sulfur on Si(111)
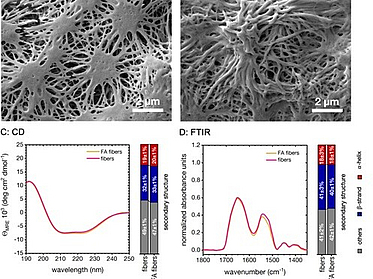
Controlling the Multiscale Structure of Nanofibrous Fibrinogen Scaffolds for Wound Healing
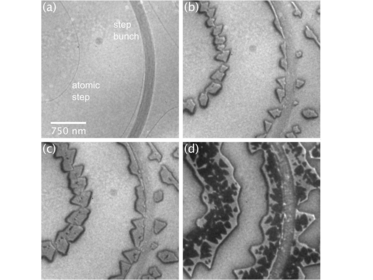
Growth and structure of ultrathin praseodymium oxide layers on ruthenium (0001)