Tobias Urbaniak, Marc Soto, Manuel Liebeke and Katharina Koschek.
Journal of Organic Chemistry (2017) 82, 4050−4055.
http://dx.doi.org/10.1021/acs.joc.6b02727
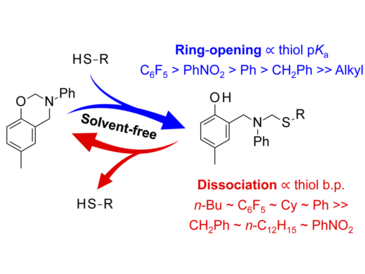
The reversible ring-opening addition and fragmentation reaction of p-cresol-based N-phenylbenzoxazine with aliphatic and aromatic thiols was investigated in solventmediated and solvent-free reactions. Independently of the used thiol, N-phenylbenzoxazine and the thiols reacted to equilibrium with comparable amounts of reactants and products in aprotic solvent, whereas in protic solvent almost full conversions were reached. In contrast, thiol reactivity was a crucial factor in solvent-free reactions yielding fast and complete conversions for a more acidic thiol and balanced equilibrium concentrations in case of thiols with high pKa values. The strong influence of thiols with low pKa values emphasizes the relevance of the protonation step in the ringopening reactions of 1,3-benzoxazines with thiols in absence of solvents where acidity predominates nucleophilicity. The reverse reactions, namely adduct dissociation and benzoxazine recovery, were successfully conducted at elevated temperatures and reduced pressure facilitated by the removal of the formed thiols yielding up to 95% recovered 1,3-benzoxazine. These results provide deeper understanding of the reversible ring-opening reaction mechanism of 1,3-benzoxazine with thiols.